
To avoid the worst effects of global warming demands an effective and economically feasible way to decrease the concentration of atmospheric CO2. Researchers in the US have developed a way to remove this greenhouse gas from the atmosphere while simultaneously producing industrial quantities of carbon nanotubes (CNTs). The low-energy, facile process, termed “C2CNT”, uses molten electrolysis to yield CNT “wools” that can be used for weaving into composites and textiles.
Led by Stuart Licht, researchers at George Washington University synthesized CNTs using a system comprising a molten lithium carbonate (Li2CO3) electrolyte, a Nichrome anode, a Monel (nickel–copper alloy) cathode, and CO2 as the only reactant. The electrolysis was performed at 770 °C under a constant current, and resulted in CNTs more than 1 µm thick and 1 mm long. This represents a 100-fold length increase compared to CNTs achieved until now.
The researchers observed by SEM that the new C2CNT method first involves the formation of a fine carbon film on the Monel cathode surface. CNTs then grow out from this graphene layer in the presence of metallic (Fe, Ni, Cr) catalysts which act as nucleation sites for dissolved CO2. The metallic particles are derived from dissolution of the Nichrome anode during electrolyte equilibrium, and results suggest that they cause CNTs to form between them and the graphene coating. This growth pattern boosts contact with the bulk electrolyte and means that CNT formation is not constrained by carbon diffusion.
As the availability of co-nucleation metallic sites is key in the production of uniform, long CNTs, the composition of the electrodes is an important factor to consider. The group’s previous approach, which yielded shorter, thicker and more convoluted CNTs, involved a zinc-coated steel cathode and a pure nickel anode, suggesting that electrode composition can tune CNT morphology.
Scalability and industrial viability
Given that the sole feedstock in the C2CNT process – CO2 – can be obtained easily from atmospheric or industrial sources, the cost is limited largely by the price of electricity. Licht and colleagues calculated the cost per ton of CNTs at $660, but this could be made even lower if solar energy is used to drive the process. Due to the electrochemical nature of the approach, its scalability is highly feasible, depending only on the size of the electrolysis chamber.
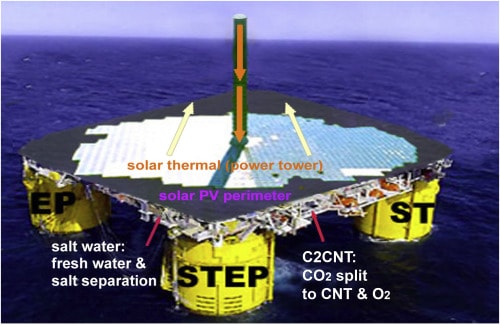
First stages of the C2CNT scaling process would include transformation of concentrated, hot sources of CO2 from cement works and power plants. This gives the further advantage of feeding co-product O2 back into the plant. Potential applications of the novel CNT wool produced include textiles, composites, and a lighter, stronger replacement for steel and aluminum. Typical sulfur and nitrogen emissions of these industrial smoke stacks could be used to produce heteroatom-doped CNT with useful properties like high electrical conductivity.
A more ambitious use of the C2CNT method would see CO2eliminated directly from the atmosphere using a combination of solar cells and focused solar thermal energy. Licht and his team estimate that dedicating less than 10% of the area of the Sahara Desert to such a project would be enough to remove all excess anthropogenic CO2 in 10 years. Alternatively, siting such facilities in open ocean areas would allow the inclusion of a water purification system as well. This approach would not only mitigate climate change and minimize the cost of CNT production, but also produce fresh water.
The work developed by Licht and his collaborators is remarkable in terms of targeting a significant societal problem with a scalable, marketable approach. Using electrochemistry, the researchers have attained conversion of a waste gas, CO2, into a valuable commodity, proving that solutions to climate change can be found in what chemistry is all about: the transformation of matter.
Full details of the research can be found in Materials Today Energy DOI: 10.1016/j.mtener.2017.07.003



