Researchers in India say they have discovered a new and simple way to probe how polymers behave at interfaces under different conditions. Their technique, which relies on magnetically polarizable nanoemulsions and visible spectroscopy, could benefit scientists working on developing colloidal formulations for improved food and cosmetic materials, drug-delivery systems and anti-bacterial surfaces, to name but a few.
Understanding how adsorbed macromolecules behave at liquid–liquid interfaces under different physico-chemicial conditions is extremely important for a number of scientific disciplines,” explains team leader John Philip of the Indira Gandhi Centre for Atomic Research. “Since the stability of a colloidal dispersion depends on the behaviour of the adsorbed moieties (for example, polymers or polyelectrolytes), studying their behaviour can also help improve the shelf life of industrial formulations.”
At the moment, researchers mainly use expensive and complex techniques like atomic-force microscopy and cryo-transmission electron microscopy to study the conformational behaviour of polymer molecules at interfaces. The new technique developed by Philip and colleague AW Zaibudeen is much simpler.
Measuring Bragg-peak position shifts
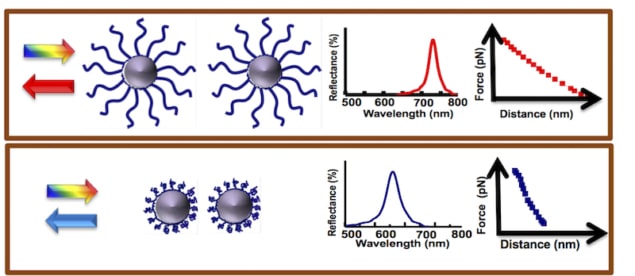
The researchers developed oil–water droplets that assemble into a periodic ID array under the influence of a weak magnetic field (with a strength of about 100 Gauss). “When we illuminate such 1D ordered structures with white light, they selectively reflect visible wavelengths when the Bragg condition is satisfied (that is, one colour is reflected),” explains Philip. “Since the droplet spacing falls in the submicron wavelength range (400–700 nm), the Bragg condition is satisfied at visible wavelengths (as opposed to X-ray wavelengths for metals) and the trick we have used in our work to probe conformational changes in polymers using this technique is quite simple.”
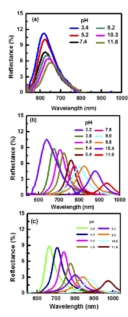
The researchers in fact attach the polymer they wish to study onto these droplets and then look at how the Bragg-peak position shifts when the droplets are placed in different environments – for example, in solutions of different pHs or ionic strengths, or at different temperatures. “All we need here is a mini fibre-optics-based spectrograph to measure the light wavelengths reflected by the drops,” says Philip.

Thanks to their technique, the researchers say they were able to observe conformational changes in the polymers under these different conditions, and determine how they behave, for example when they are extended and stretched or when they are collapsed.
Important for understanding biological processes
As well as being important for developing colloidal formulations for industrial applications, as mentioned, the way polyelectrolytes behave under various environmental conditions is also fundamentally important for understanding biological processes such as protein folding and DNA condensation, he states. This is because a polyelectrolyte adsorbed at a liquid–liquid interface mimics the in vivo conditions of charged macromolecules at cell–fluid interfaces.
The team, reporting its results in the Journal of Molecular Liquids doi.org/10.1016/j.molliq.2017.12.090, says that it is now busy developing a portable spectrograph that has magnetic field and temperature control options. “We are also trying to design a microfluidic set up incorporating the above features,” Philip tells nanotechweb.org.



