The energy storage capacity of lithium-ion batteries can be significantly increased thanks to an anode made from folded graphene. A device with a mass loading of 5 mg/cm2 of the graphene has an areal capacity of more than 4 mAh/cm2, which is well above that of commercial graphite anodes, and it can withstand at least 500 cycles of battery charging/discharging without any loss in its performance. The simple folding strategy could easily work for other electrode materials too, say the researchers in the Republic of Korea who report on this work.
“Small-sized, high-energy density batteries will increasingly be needed in applications such as portable electronics as well as in large-scale energy-storage devices, such as electric vehicles,” explains co-team leader Rodney Ruoff and Soojin Park of the Institute of Basic Science (IBS) in Ulsan and the Ulsan National Institute of Science and Technology (UNIST) and the Center for Multidimensional Carbon Materials (an Institute for Basic Science at UNIST), South Korea. “To reduce battery size, electrodes with high areal and volumetric capacities are crucial, but the problem here is that much space in these structures is currently being ‘wasted’ by inactive materials or by pores that are present in traditional electrode configurations.
“These electrodes can be made of graphene, for example, and contain freestanding graphene composite films comprised of densely stacked layers but they suffer from low areal energy storage because of poor electron/ion transport kinetics. Our folded electrode, which is also made from graphene composite films, shows much improved electron/ion transport and thus a high areal capacity over multiple recharging cycles.”
New paths and channels
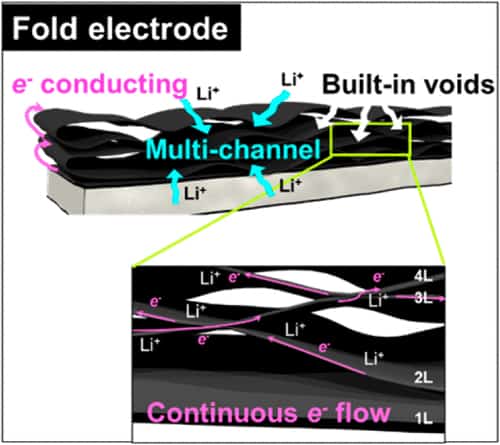
Compared to traditional thick film electrodes, the paper-like folded electrode contains many continuous layered films. The folds at the edges of these films provide new paths through which free electrons can flow and the small gaps that exist between the folds form multiple channels through which lithium ions can diffuse. These paths and channels significantly increase areal/volumetric energy density. Indeed, a folded electrode made of tin oxide/graphene contains as much as 5 mg/cm2 of active material while being just 20 microns thick. It also has a high areal density of 4.15 mAh/cm2, which is much higher than that of commercial graphite anodes that have areal capacities of between just 2.50 and 3.50 mAh/cm2.
The folded electrode is stable over 500 cycles of battery charging/discharging and boasts improve rate capability compared to thick graphene electrodes with the same mass loading of active material, but without folds.
No binders, carbon additives or metal current collectors required
“Folding the graphene electrode several times is in fact a way to thicken the electrode,” Ruoff and Park tell nanotechweb.org. “By increasing the electrode thickness in this way, while maintaining the electron transport efficiency of the electrode along the folds, is the reason why it has such as high areal/volumetric energy density.”
And that is not all: the researchers say that they can assemble their freestanding electrodes in a battery cell without having to resort to traditional methods, such as slurry coating, for making these electrodes. They can also completely do without binders, carbon additives and metal current collectors.
Lighter battery and higher energy density
“Our approach greatly lowers the weight of the final battery and increases its energy density,” explains team member Bin Wang. “For example, in a full cell (containing both an anode and cathode) made with the standard graphite anode and a standard LiCoO2 cathode, the energy density is around 271 Wh/kg, but in our system of folded graphene anode and the same LiCoO2 cathode, it goes up to 452 Wh/kg. Such a cell is also stable over 300 charge/discharge cycles and has an areal capacity as high as 2.84 mAh/cm2.”
The good news is that the folding technique can be extended to other types of active materials and even other kinds of rechargeable batteries, not just Li-ion ones. What is more, new more intricate folding configurations, and not just simple back and forth folding as was done in this study, could also be possible, says Ruoff.
“In fact, the folding approach may even be useful for electrodes in supercapacitors,” adds team member Jaegeun Ryu.
“And in a separate research project on composite materials, we are trying to employ this type of folding to make thick samples from microscale films, including those made of composite materials. Such a bottom-up approach could be useful for controlling the structure and properties of the as-built materials,” adds Wang>
The new folded graphene anode is detailed in ACS Nano DOI: 10.1021/acsnano.7b08489.