How did complex carbon-based compounds form in in the Universe, and in particular in our galaxy? New experiments that retrace the synthesis of polycyclic aromatic hydrocarbons (PAHs), such as pyrene, could help answer this question. The work could also help explain how more complex PAHs, and eventually 2D graphene-type structures formed from pyrene thanks to molecular mass growth.
PAHs (which are organic molecules comprising fused benzene rings) along with alkylated (methyl, ethyl), ionized, (de)hydrogenated and protonated counterparts may make up 20% of all the carbon in our galaxy. PAHs have been detected in some carbonaceous meteorites such as Allende and Murchison, which suggests that they come from deep space. Thanks to carbon-13/carbon-12 and deuterium/hydrogen isotopic analyses, researchers believe that molecular mass growth processes allow higher molecular weight PAHs to form from lower-weight ones. The main astrochemical reactions at play here might be similar to those occurring in combustion processes in vehicle engines and in the formation of soot particles.
To find out how PAHs develop in space, researchers led by Alexander Mebel at Florida International University and Ralf Kaiser of the University of Hawaii, synthesized these molecules by building them up one ring at a time. They studied the chemical reactions that begin when a complex hydrocarbon, the 4-phenanthrenyl radical (which has a molecular structure that includes a sequence of three rings and contains 14 carbon atoms and nine hydrogen atoms) combines with acetylene (which has two carbon atoms and two hydrogen atoms).

Intermediate reaction steps
The researchers performed their experiments at the Advanced Light Source (ALS) at the Berkeley Laboratory. They injected the gas mixture into a microreactor and heated the sample to temperatures as high as those that exist around stars. They then focused a VUV light beam from the ALS synchrotron onto the heated gas mixture to ionize the molecules in the sample.
The team then proceeded to analyse the chemical reactions taking place using a detector that measures the different arrival time of particles created after ionization. These times reveal the signature of the parent molecules and, together with theory calculations, allow the researchers to determine the intermediate steps in the reactions.
Towards an understanding of the molecular carbon budget in our galaxy
The experiments indeed show that a four-ringed molecule (pyrene) can be produced from a three-ringed one (phenanthrene). “Large chains of ringed molecules and ultimately 2D graphene-type structures might form via the same processes,” says Kaiser.
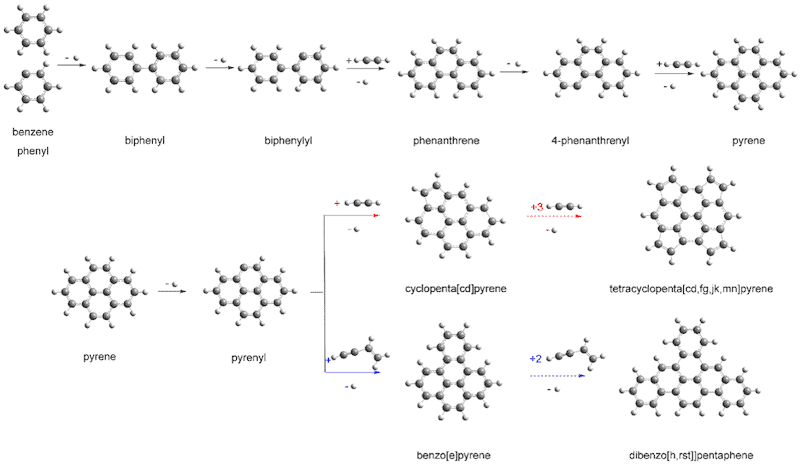
This result brings us closer to an understanding of the molecular carbon budget in our galaxy and the fundamental molecular level processes of synthesizing PAHs, say the researchers, reporting their work in Nature Astronomy doi:10.1038/s41550-018-0399-y. “This is how we believe some of the first carbon-based structures evolved in the Universe,” adds Musahid Ahmed of the Berkeley Laboratory. “From 2D graphene you can then get graphite, and the evolution of more complex chemistry begins, he says.