A new, non-invasive skin patch made from thin-film graphene could measure glucose levels without the need for a finger-prick blood test. The device works by drawing out glucose from the fluid between cells across hair follicles, which are individually accessed via a miniaturized pixel array platform using a small electric current, in a process called electroosmotic extraction. Readings can be taken every 10 to 15 minutes over several hours and the data wirelessly transmitted to a mobile device, such as a smartphone or watch.
Diabetes is a serious worldwide health problem that is set to become even worse. The World Health Organization estimates that the number of people suffering from diabetes will increase to 366 million in 2030. This number was 171 million in 2000.
Diabetics regularly need to monitor their blood glucose levels, and they usually do this with a finger-prick test, which is uncomfortable to say the least. Researchers have recently developed a variety of non-invasive alternatives based on detecting glucose in sweat, tears or saliva but these techniques have their limits. For one, the levels of glucose detected can vary significantly, and secondly, many still require calibration with a conventional finger-stick.
Until now, the only technology to provide non-invasive, continuous glucose monitoring was the Gluco Watch Biographer, which received Food and Drug Administration approval in 2001. This watch works using reverse iontophoresis to extract interstitial fluid through the skin via electroosmosis. In this technique, a small electric field is applied across the skin, causing ions to flow. Since the skin has a net negative charge under normal physiological conditions, interstitial fluid flows from within the skin towards a cathode on the skin surface, where glucose is then detected and analysed.
Calibration via a blood sample not required
The problem with the watch is that, again, a finger-stick calibration is required, mainly because glucose is extracted indiscriminately and variably across a relatively large area (>3 cm2) of skin and is considerably diluted before it is analysed. “The device therefore does not exploit the fact that most of the electroosmotic flow during iontophoresis follows low-resistance preferential pathways associated primarily with hair follicles,” say Adelina Ilie and colleagues of the University of Bath in the UK.
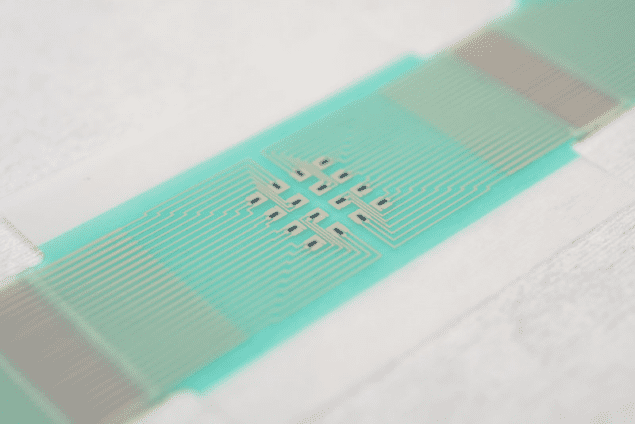
The researchers have now designed a non-invasive adhesive patch containing a sensor array that can operate on a small area over an individual hair follicle. “This significantly reduces inter- and intra-skin variability in glucose extraction,” they say, “and increases the accuracy of the measurements taken such that calibration via a blood sample is not required.”
A miniature, individual pixel of such an array contains the following: a glucose oxidase-bearing hydrogel reservoir into which glucose is extracted through skin; an electrochemical glucose sensor (made of a graphene-based film or graphene ink); and miniaturized electrodes on a flexible substrate. Ilie and her team used graphene as their material of choice, thanks to its high mechanical strength, high conductivity, low capacitance, large surface area and the fact that it can be patterned and integrated into a device using standard microfabrication techniques. It is also low cost and environmentally friendly.
Monitoring glucose levels across the hypo- to hyper-glycaemic range
The glucose extracted via reverse iontophoresis reacts with glucose oxidase to produce hydrogen peroxide, which is then detected by the electrochemical sensor. One important aspect of the device is that the active area of the pixel device (into which fluid is extracted) allows it to access a single follicular pathway. “If we assume that a person has an average follicular density of 27 follicles per cm2 (for instance, on areas such as the arms and thighs), an active area of 2–6 mm2 maximizes the probability of hitting a single follicle in a randomly positioned, untargeted measurement,” say the researchers.
The team tested its patch on both pig skin (which is an excellent model for the human skin barrier) and on healthy human volunteers and found that it could accurately monitor glucose levels across the hypo- to hyper-glycaemic range in diabetic patients over six hours.
The researchers, reporting their work in Nature Nanotechnology doi:10.1038/s41565-018-0112-4, say that they would now like to further improve the design of their approach. They would also like to optimize the number of sensors in the array so that it can operate over a full 24-hour period. Finally, they would like to undertake a number of key clinical trials.



